Direct C–H aminocarbonylation of N-heteroarenes with isocyanides under transition metal-free conditions†
Abstract
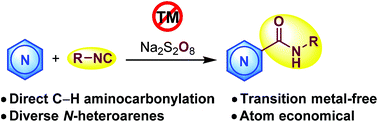
A C–C bond forming amide synthesis through direct C–H aminocarbonylation of N-heteroarenes with isocyanides was developed. The reaction was mediated by an inorganic persulfate salt under transition metal-free conditions. Mechanistic studies suggested a radical pathway for this reaction without the participation of H2O and O2. This method also showed merits of substrate availability, easy operation and atom economy. It provided an efficient route for straightforward synthesis of N-heteroaryl amides.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...