Conversion of esters to thioesters under mild conditions†
Abstract
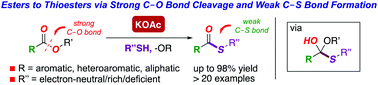
We report conversion of esters to thioesters via selective C–O bond cleavage/weak C–S bond formation under transition-metal-free conditions. The method is notable for a general and practical transition-metal-free system, broad substrate scope and excellent functional group tolerance. The strategy was successfully deployed in late-stage thioesterification, site-selective cross-coupling/thioesterification/decarbonylation and easy-to-handle gram scale thioesterification. Selectivity and computational studies were performed to gain insight into the formation of weak C–S bonds by C–O bond cleavage, which contrasts with the traditional trend of nucleophilic additions to carboxylic acid derivatives.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...