Copper catalyzed five-component domino strategy for the synthesis of nicotinimidamides†
Abstract
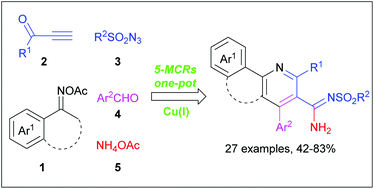
A library of medicinally and synthetically important nicotinimidamides was synthesized by a copper-catalyzed multicomponent domino reaction of oxime esters, terminal ynones, sulfonyl azides, aryl aldehydes and acetic ammonium. Its synthetic pathway involves the formation of a highly reactive N-sulfonyl acetylketenimine, characterized by high selectivity, combinations of potential nucleophiles and electrophiles, mild reaction conditions and a wide substrate scope, and is a rare five-component example of a CuAAC/ring-opening reaction.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...