Visible-light-mediated cascade cyanoalkylsulfonylation/cyclization of alkynoates leading to coumarins via SO2 insertion†
Abstract
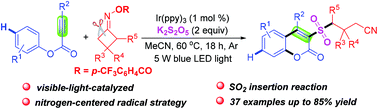
A visible-light-mediated tandem cyanoalkylsulfonylation/cyclization of alkynoates with cycloketone oxime compounds for the preparation of 3-cyanoalkylsulfonylcoumarins via SO2 insertion is reported. The difunctionalization of carbon–carbon triple bonds includes a radical mechanism and involves the formation of an iminyl radical, ring-opening of the cycloketone, insertion of SO2, addition of the sulfonyl radical to carbon–carbon triple bonds, ipso-cyclization and ester migration.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...