Copper-catalyzed ortho-selective direct sulfenylation of N-aryl-7-azaindoles with disulfides†
Abstract
A copper-catalyzed direct C–H chalcogenation of N-aryl-azaindoles with disulfides is described. This transformation was performed using Earth abundant Cu(OAc)2 as a catalyst, benzoic acid as an additive, air as a terminal oxidant, and readily available diaryl and dialkyldisulfides (or diselenide) as chalcogenation reagents. High functional group tolerance and excellent regioselectivity are demonstrated by the efficient preparation of a wide range of ortho-sulfenylation-7-azaindoles.
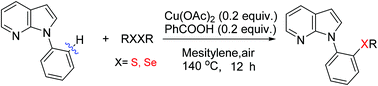
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...