Synthesis and reactivity of hydroindole enelactams leading to densely functionalized scaffolds†
Abstract
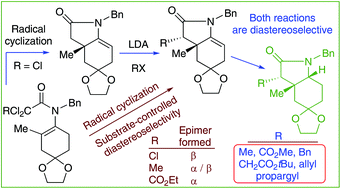
The 5-endo-trig radical cyclization of N-benzyl-N-[(2-substituted)cycloalkenyl] trichloroacetamides (tetrasubstituted enamides) using Bu3SnH and AIBN is a reliable synthetic procedure giving access to 3a-methyl- and 3a-methoxycarbonyl enelactams. The substrate-controlled diastereoselective enolate alkylation of these enelactams resulted in the synthesis of a set of 3-substituted derivatives that upon reduction furnished polyfunctionalized cis-octahydroindoles. The latter building blocks, which embody three consecutive stereocenters at C-3, C-3a, and C-7a, were also synthesized through an initial reductive radical cyclization using (carbo-substituted)dichloroacetamides.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...