A divergent chiron approach for the first total synthesis of (+)-pseudonocardide A, (+)-pseudonocardide C and an epimer of ent-pseudonocardide D†
Abstract
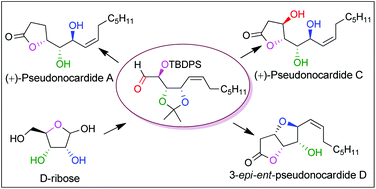
A concise and divergent chiron approach for the first total synthesis of (+)-pseudonocardide A, (+)-pseudonocardide C and an epimer of ent-pseudonocardide D is reported starting from D-ribose. The salient features of this synthesis are a highly Z-selective Wittig olefination, one-pot formation of γ-butyrolactone and γ-butenolide through [1,4] O-to-O silyl migration followed by lactonization and an intramolecular oxa-Michael reaction.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...