Divorce in the two-component BVMO family: the single oxygenase for enantioselective chemo-enzymatic Baeyer–Villiger oxidations†
Abstract
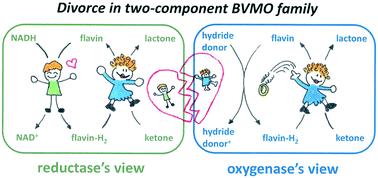
Two-component flavoprotein monooxygenases consist of a reductase and an oxygenase enzyme. The proof of functionality of the latter without its counterpart as well as the mechanism of flavin transfer remains unanswered beyond doubt. To tackle this question, we utilized a reductase-free reaction system applying purified 2,5-diketocamphane-monooxygenase I (2,5-DKCMO), a FMN-dependent type II Baeyer–Villiger monooxygenase, and synthetic nicotinamide analogues (NCBs) as dihydropyridine derivatives for FMN reduction. This system demonstrated the stand-alone quality of the oxygenase, as well as the mechanism of FMNH2 transport by free diffusion. The efficiency of this reductase-free system strongly relies on the balance of FMN reduction and enzymatic (re)oxidation, since reduced FMN in solution causes undesired side reactions, such as hydrogen peroxide formation. Design of experiments allowed us to (i) investigate the effect of various reaction parameters, underlining the importance to balance the FMN/FMNH2 cycle, (ii) optimize the reaction system for the enzymatic Baeyer–Villiger oxidation of rac-bicyclo[3.2.0]hept-2-en-6-one, rac-camphor, and rac-norcamphor. Finally, this study not only demonstrates the reductase-independence of 2,5-DKCMO, but also revisits the terminology of two-component flavoprotein monooxygenases for this specific case.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC

 Please wait while we load your content...
Please wait while we load your content...