Value-added chemicals from biomass-derived furans: radical functionalisations of 5-chloromethylfurfural (CMF) by metal-free ATRA reactions†
Abstract
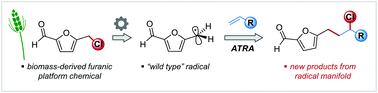
Biomass-derived 5-chloromethylfurfural (CMF), a congener of the well-known carbohydrate-based platform chemical 5-hydroxymethylfurfural (HMF), can efficiently be functionalised by radical transformations of its benzylic chloromethyl group. We report here the first examples of these radical reactions by way of metal-free, triethylborane/oxygen-induced atom transfer radical addition (ATRA) reactions between CMF and styrenes, which proceed with high yield and selectivity. The key intermediate, the 2-formyl-5-furfuryl radical derived from CMF, and its radical addition reactions were studied with regard to its electronic structure, i.e. spin density distribution and frontier molecular orbitals based on the NBO ansatz and activation barriers of the addition step using DFT and post-HF methods.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...