Synthesis and immunological evaluation of the unnatural β-linked mucin-1 Thomsen–Friedenreich conjugate†
Abstract
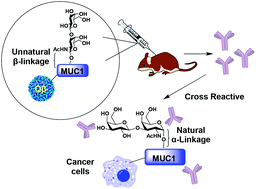
MUC1 glycopeptides are attractive antigens for anti-cancer vaccine development. One potential drawback in using the native MUC1 glycopeptide for vaccine design is the instability of the O-glycosyl linkage between the glycan and the peptide backbone to glycosidase. To overcome this challenge, a MUC1 glycopeptide mimic has been synthesized with the galactose-galactosamine disaccharide linked with threonine (Thomsen–Friedenreich or Tf antigen) through an unnatural β-glycosyl bond. The resulting MUC1-β-Tf had a much-enhanced stability toward a glycosidase capable of cleaving the glycan from the corresponding MUC1 glycopeptide with the natural α-Tf linkage. The MUC1-β-Tf was subsequently conjugated with a powerful carrier bacteriophage Qβ. The conjugate induced high levels of IgG antibodies in clinically relevant human MUC1 transgenic mice, which cross-recognized not only the natural MUC1-α-Tf glycopeptide but also MUC1 expressing tumor cells, supporting the notion that a simple switch of the stereochemistry of the glycan/peptide linkage can be a strategy for anti-cancer vaccine epitope design for glycopeptides.
- This article is part of the themed collection: Glycosylation: New methodologies and applications


 Please wait while we load your content...
Please wait while we load your content...