Metal-free tandem reactions of 2-iodoaryl ynones with sodium azide for the synthesis of benzoisoxazole containing 1,2,3-triazoles†
Abstract
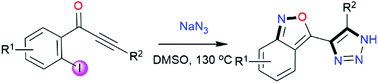
A metal-free approach has been developed for the synthesis of benzo[c]isoxazole (anthranils) containing 1,2,3-triazoles. The reaction proceeded efficiently through a [3 + 2] azide–alkyne cycloaddition, SNAr azidation and denitrogenative cyclization sequence. The metal-free protocol enabled effective construction of one N–O and three C–N bonds in one pot. In addition, the synthetic utility of the current methodology was further demonstrated by late stage modification of the obtained products.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...