Catalytic mechanism of the colistin resistance protein MCR-1†
Abstract
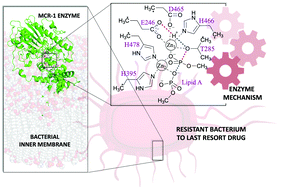
The mcr-1 gene encodes a membrane-bound Zn2+-metalloenzyme, MCR-1, which catalyses phosphoethanolamine transfer onto bacterial lipid A, making bacteria resistant to colistin, a last-resort antibiotic. Mechanistic understanding of this process remains incomplete. Here, we investigate possible catalytic pathways using DFT and ab initio calculations on cluster models and identify a complete two-step reaction mechanism. The first step, formation of a covalent phosphointermediate via transfer of phosphoethanolamine from a membrane phospholipid donor to the acceptor Thr285, is rate-limiting and proceeds with a single Zn2+ ion. The second step, transfer of the phosphoethanolamine group to lipid A, requires an additional Zn2+. The calculations suggest the involvement of the Zn2+ orbitals directly in the reaction is limited, with the second Zn2+ acting to bind incoming lipid A and direct phosphoethanolamine addition. The new level of mechanistic detail obtained here, which distinguishes these enzymes from other phosphotransferases, will aid in the development of inhibitors specific to MCR-1 and related bacterial phosphoethanolamine transferases.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...