Catalyst-free synthesis of 3,1-benzoxathiin-4-ones/1,3-benzodioxin-4-ones†
Abstract
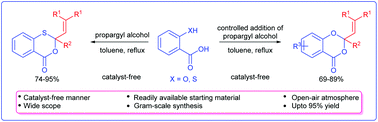
An unambiguous and precise method for the synthesis of 3,1-benzoxathiin-4-ones/1,3-benzodioxin-4-ones by the reaction of propargylic alcohols and salicylic/thiosalicylic acids under a catalyst-free and open-air atmosphere is described. This strategy is found to be quite general using various 2-mercapto and 2-hydroxybenzoic acids providing benzoxathiinones/benzodioxinones in good yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...