One step stereoselective synthesis of oxazoline-fused saccharides and their conversion into the corresponding 1,2-cis glycosylamines bearing various protected groups†
Abstract
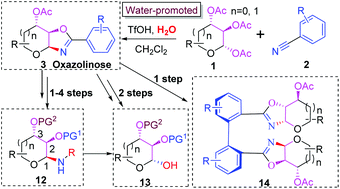
Herein we disclosed a straightforward synthesis of oxazoline-fused saccharides (oxazolinoses) from peracetylated saccharides and benzonitriles under acidic conditions with stoichiometric amounts of water. The density functional theory (DFT) calculations have revealed the origin of the stereoselectivity and the key role of water in promoting the departure of the acetyl group at C-2. The resulting oxazolinoses can be concisely converted into the corresponding 1,2-cis glycosylamines bearing various protected groups, allowing the access to schisandrin derivatives.


 Please wait while we load your content...
Please wait while we load your content...