Probing the anomeric effect and mechanism of isomerization of oxazinane rings by DFT methods†
Abstract
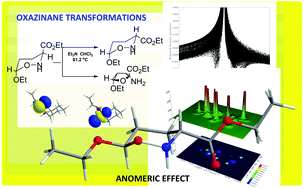
Mechanistic studies of the thermal amine-promoted isomerization of oxazinane rings by DFT methods showed that the isomerization proceeds through abstraction of the C-3 hydrogen atom by the amine nitrogen atom followed by its re-recruitment from C-3 that helps the oxazinane ring to avoid breaking, leading to the same or an isomeric conformer. Calculations also provided evidence that steric effects are responsible for the breaking of the O–N bond in the transition state of the thermal amine-promoted transformations of oxazinane rings, leading to the transformation of the 6-membered ring to a 5-membered ring. Extensive computational studies of the origin of the anomeric effect in the di-substituted oxazinane rings, bearing the EtO substituent at C-6 and CO2Et at C-3, and a series of analogous tetrahydro-2H-pyran ring conformers, revealed that the conformational preferences in both series of compounds are tuned by the balance of non-covalent (weak vDW, dipole–dipole, electrostatic forces, hydrogen bonding) steric effects and hyperconjugative interactions.


 Please wait while we load your content...
Please wait while we load your content...