Thermal switches between delayed fluorescence and persistent phosphorescence based on a keto-BODIPY electron acceptor†
Abstract
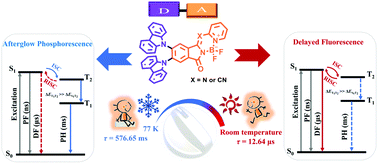
A new type of twisted donor–acceptor molecular material 3a and 3b containing carbazole as an electron donor and keto-BODIPY bearing keto-isoindolinyl and pyridyl subunits as an acceptor has been prepared and characterized. Chemical modifications at the meso-position of keto-BODIPY with a nitrogen atom and a cyano group enhance the electron withdrawing ability and cause the emission color change from blue to yellow and red. Steady-state absorption and emission spectra of the two compounds show a strong intramolecular charge transfer (ICT) character. Time-resolved emission spectra and transient decay curves of 3a and 3b show efficient delayed fluorescence with a lifetime of 12.64 μs for 3a and 16.59 μs for 3b at room temperature, whereas persistent phosphorescence with a lifetime of 576.65 ms for 3a and 273.76 ms for 3b was obviously detected at 77 K. These photophysical behaviors have been fully revealed via X-ray diffraction analysis and theoretical calculations, and thus attributed to the hybridized local and charge-transfer (HLCT) states and increased spin–orbital coupling (SOC) strength by mixed n → π* and π → π* transitions involving heteroatom lone pairs and the π-conjugated skeleton, respectively.


 Please wait while we load your content...
Please wait while we load your content...