Rhodium(iii)-catalyzed oxidative alkylation of N-aryl-7-azaindoles with cyclopropanols†
Abstract
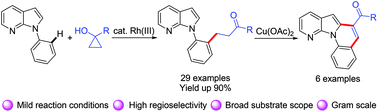
An efficient Rh(III)-catalyzed C–H oxidative alkylation of N-aryl-7-azaindoles with cyclopropanols by merging tandem C–H and C–C cleavage was developed. This transformation features mild reaction conditions, high regioselectivity, and excellent functional group compatibility. The resulting β-aryl ketone derivatives can be readily transformed into 7-azaindole-containing π-extended polycyclic heteroarenes.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...