Metal-catalyzed C–S bond formation using sulfur surrogates†
Abstract
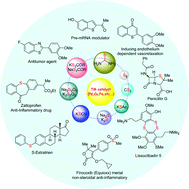
Sulfur-containing compounds are present in a wide range of biologically important natural products, drugs, catalysts, and ligands and they have wide applications in material chemistry. Transition metal-catalyzed C–S bond-forming reactions have successfully overcome the obstacles associated with traditional organosulfur compound syntheses such as stoichiometric use of metal-catalysts, catalyst-poisoning and harsh reaction conditions. One of the key demands in metal-catalyzed C–S bond-forming reactions is the use of an appropriate sulfur source due to its odor and availability. The unpleasant odor of many organic sulfur sources might be one of the reasons for the metal-catalyzed C–S bond-forming reactions being less explored compared to other metal-catalyzed C–heteroatom bond-forming reactions. Hence, employing an appropriate sulfur surrogate in the synthesis of organosulfur compounds in metal-catalyzed reactions is still of prime interest for chemists. This review explores the recent advances in C–S bond formation using transition metal-catalyzed cross-coupling reactions and C–H bond functionalization using diverse and commercially available sulfur surrogates. Based on the different transition metal-catalysts, this review has been divided into three major classes namely (1) palladium-catalyzed C–S bond formation, (2) copper-catalyzed C–S bond formation, and (3) other metal-catalyzed C–S bond formation. This review is further arranged based on the different sulfur surrogates. Also, this review provides an insight into the growing opportunities in the construction of complex organosulfur scaffolds covering natural product synthesis and functional materials.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...