Copper(ii)-catalysed direct C3–H esterification of indoles assisted by an N,N-bidentate auxiliary moiety†
Abstract
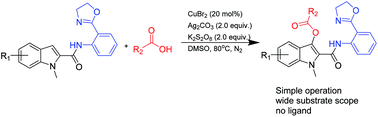
The regioselective direct C3-esterification of indoles with OXA is developed in an efficient reaction with carboxylic acids using the catalyst CuBr2 and oxidants Ag2CO3 and K2S2O8. The simple experimental procedure is proved to be broadly applicable to a range of substrates, including aromatic and aliphatic acids, and the corresponding products were obtained in good yields up to 87%. At the same time, it provides a valuable approach to produce C3-benzyl derivatives of indoles through reaction with benzyl carboxylic acid under the same reaction conditions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...