Generation and trapping of electron-deficient 1,2-cyclohexadienes. Unexpected hetero-Diels–Alder reactivity†
Abstract
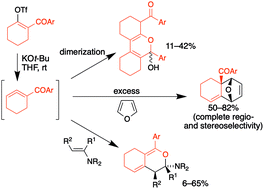
Keto-substituted 1,2-cyclohexadienes were generated by base-mediated (KOt-Bu) elimination, and found to dimerize via an unprecedented formal hetero-Diels–Alder process, followed by hydration. These highly reactive cyclic allene intermediates were also trapped in Diels–Alder reactions by furan, 2,5-dimethylfuran, or diphenylisobenzofuran to afford cycloadducts with high regio- and diastereoselectivity, and could also be intercepted in a hetero-Diels–Alder process with enamine dienophiles. Endo/exo stereochemistry was unambiguously determined via X-ray crystallography in the case of nitrile-substituted 1,2-cyclohexadiene. DFT calculations indicate that the novel hetero-Diels–Alder processes observed with these allenes occur via a concerted asynchronous cycloaddition mechanism.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...