2-Nitroallyl carbonate-based green bifunctional reagents for catalytic asymmetric annulation reactions†
Abstract
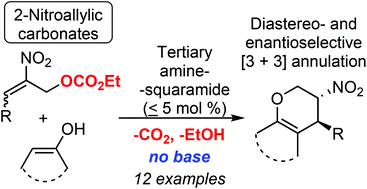
2-Nitroallylic carbonates, a new class of “green” 1,3-bielectrophilic reagents for organic synthesis and catalysis, have been prepared. The bifunctional tertiary amine-catalyzed asymmetric [3 + 3] annulations of cyclic enols with these reagents occur much faster than corresponding reactions with 2-nitroallylic esters and produce no acidic by-products poisoning the catalyst. Furthermore, 2-nitroallylic carbonates enable highly enantioselective one-pot synthesis of a variety of fused dihydropyrane derivatives from available precursors bearing pharmacophoric fragments.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...