Hancockiamides: phenylpropanoid piperazines from Aspergillus hancockii are biosynthesised by a versatile dual single-module NRPS pathway†
Abstract
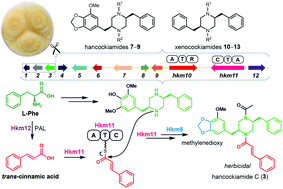
The hancockiamides are an unusual new family of N-cinnamoylated piperazines from the Australian soil fungus Aspergillus hancockii. Genomic analyses of A. hancockii identified a biosynthetic gene cluster (hkm) of 12 genes, including two single-module nonribosomal peptide synthetase (NRPS) genes. Heterologous expression of the hkm cluster in A. nidulans confirmed its role in the biosynthesis of the hancockiamides. We further demonstrated that a novel cytochrome P450, Hkm5, catalyses the methylenedioxy bridge formation, and that the PAL gene hkm12 is dispensable, but contributes to increased production of the cinnamoylated hancockiamides. In vitro enzymatic assays and substrate feeding studies demonstrated that NRPS Hkm11 activates and transfers trans-cinnamate to the piperazine scaffold and has flexibility to accept bioisosteric thienyl and furyl analogues. This is the first reported cinnamate-activating fungal NRPS. Expression of a truncated cluster lacking the acetyltransferase gene led to seven additional congeners, including an unexpected family of 2,5-dibenzylpiperazines. These pleiotropic effects highlight the plasticity of the pathway and the power of this approach for accessing novel natural product scaffolds.


 Please wait while we load your content...
Please wait while we load your content...