Silver-catalyzed tandem cycloisomerization/hydroarylation reactions and mechanistic investigations for an efficient access to 1,2-dihydroisoquinolines†‡
Abstract
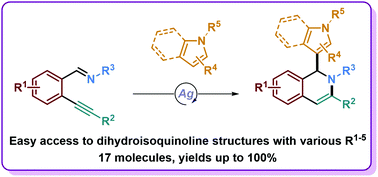
An efficient silver-catalyzed tandem reaction for the formation of 1,2-dihydroisoquinoline derivatives is herein reported. Highly functionalized multiheterocyclic scaffolds are accessible in a straightforward manner using readily accessible starting materials under mild conditions. This methodology offers an attractive route for the synthesis and development of a biologically relevant new heterocyclic pharmacophore, merging the biological activities of isoquinolines with those of various nitrogen-containing heterocycles (indoles, pyrroles) incorporated during the tandem reaction. Mechanistic investigations were also conducted along with a large scope and limitation study, modifying various sites of this pharmacophore.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...