Insertion of Pro-Hyp-Gly provides 2 kcal mol−1 stability but attenuates the specific assembly of ABC heterotrimeric collagen triple helices†
Abstract
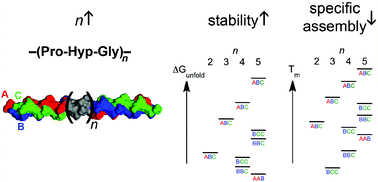
Collagen is a major structural component of the extracellular matrix and connective tissue. The key structural feature of collagen is the collagen triple helix, with a Xaa-Yaa-Gly (glycine) repeating pattern. The most frequently occurring triplet is Pro (proline)-Hyp (hydroxyproline)-Gly. The reversible thermal folding and unfolding of a series of heterotrimeric collagen triple helices with varying number of Pro-Hyp-Gly triplets were monitored by circular dichroism spectroscopy to determine the unfolding thermodynamic parameters Tm (midpoint transition temperature), ΔHTm (unfolding enthalpy), and ΔGunfold (unfolding free energy). The Tm and ΔGunfold of the heterotrimeric collagen triple helices increased with increasing number of Pro-Hyp-Gly triplets. The ΔGunfold increased by 2.0 ± 0.2 kcal mol−1 upon inserting one Pro-Hyp-Gly triplet into all three chains. The Tm difference between the most stable ABC combination and the second most stable BCC combination decreased with increasing number of Pro-Hyp-Gly triplets, even though the ΔGunfold difference remained the same. These results should be useful for tuning the stability of collagen triple helical peptides for hydrogel formation, recognition of denatured collagen triple helices as diagnostics and therapeutics, and targeted drug delivery.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...