Synthesis of the hyper-branched core tetrasaccharide motif of chloroviruses†
Abstract
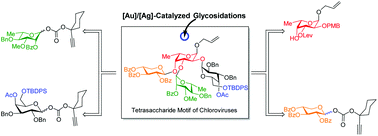
Chemical synthesis of complex oligosaccharides, especially those possessing hyper-branched structures with one or multiple 1,2-cis glycosidic bonds, is a challenging task. Complementary reactivity of glycosyl donors and acceptors and proper tuning of the solvent/temperature/activator coupled with compromised glycosylation yields for sterically congested glycosyl acceptors are among several factors that make such syntheses daunting. Herein, we report the synthesis of a semi-conserved hyper-branched core tetrasaccharide motif from chloroviruses which are associated with reduced cognitive function in humans as well as in mouse models. The target tetrasaccharide contains four different sugar residues in which L-fucose is connected to D-xylose and L-rhamnose via a 1,2-trans glycosidic bond, whereas with the D-galactose residue is connected through a 1,2-cis glycosidic bond. A thorough and comprehensive study of various accountable factors enabled us to install a 1,2-cis galactopyranosidic linkage in a stereoselective fashion under [Au]/[Ag]-catalyzed glycosidation conditions en route to the target tetrasaccharide motif in 14 steps.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...