Highly diastereo- and enantioselective organocatalytic synthesis of trifluoromethylated erythritols based on the in situ generation of unstable trifluoroacetaldehyde†‡
Abstract
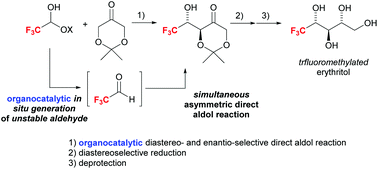
Thus far, only a few methods for the asymmetric synthesis of erythritols bearing a trifluoromethyl group have been developed, and these methods present serious disadvantages such as the requirement of multiple steps for the preparation of their starting materials, low stereoselectivity, and the use of highly toxic reagents. Herein, we have developed a highly diastereo- and enantioselective organocatalytic method to synthesise erythritols bearing a trifluoromethyl group using (1) a commercially available organocatalyst to produce unstable trifluoroacetaldehyde in situ from its corresponding hemiacetal, followed by the simultaneous asymmetric carbon–carbon bond-forming reaction of the organocatalyst with an in situ-generated chiral enamine derived from 2,2-dimethyl-1,3-dioxane-5-one to obtain the corresponding aldol product in good yield (65–80%) with high diastereoselectivity (up to 94% de) and excellent enantioselectivity (up to >98% ee), (2) the highly diastereoselective reduction of the ketone moiety in the aldol product (up to 98% de), and (3) the deprotection of the acetal moiety.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...