C(sp2)–H functionalization in non-aromatic azomethine-based heterocycles
Abstract
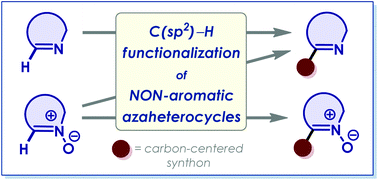
Direct C(sp2)–H functionalization of the endocyclic azomethine and aldonitrone moieties in non-aromatic azaheterocycles has established itself as a promising methodology over the last decade. Transition metal-catalyzed cross-coupling reactions, α-metalation–electrophile quenching protocols, and (metal-free) nucleophilic substitution of hydrogen reactions (SNH) are the major routes applied on cyclic imines and their derivatives. In this overview, we show the tangible progress made in this area during the period from 2008 to 2020.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...