Possible competitive modes of decarboxylation in the annulation reactions of ortho-substituted anilines and arylglyoxylates†
Abstract
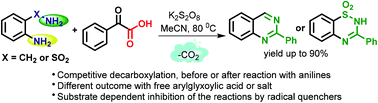
Annulation reactions of ortho-substituted anilines and arylglyoxylates in the presence of K2S2O8 at 80 °C under metal-free neutral conditions have been investigated, which extended a platform for the tandem synthesis of nitrogen heterocycles. While arylglyoxylic acids are known to undergo decarboxylation to form an acyl radical in the presence of K2S2O8 and used in the Minisci acylation of electron-deficient (hetero)aromatics, their reactions with electron-rich ortho-substituted anilines to form nitrogen heterocycles have recently been studied. Depending upon the experimental conditions used in the reactions, the mechanism of the formation of heterocycles involving reactions of an acyl radical or aryl iminocarboxylic acids has been postulated. Given the subtle understanding of the mechanisms of annulation reactions of 2-substituted anilines and arylglyoxylates in the presence of K2S2O8, an extensive mechanistic investigation was undertaken. In the current study, the various mechanistic pathways including the generation of acyl, imidoyl, aminal, and N,O-hemiketal radicals have been postulated based on different possible decarboxylation modes. Some of the proposed intermediates are supported based on the available analytical data. The protocol uses a single, inexpensive reagent K2S2O8, which offers not only transition-metal-free conditions but also serves as the reagent for the key decarboxylation step. Taken together, this study complements the current development of the annulation reactions of 2-substituted anilines and arylglyoxylates in terms of synthesis and mechanistic understanding.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...