Electrochemical gelation of quantum dots using non-noble metal electrodes at high oxidation potentials†
Abstract
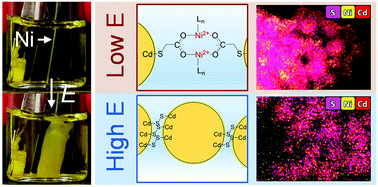
Relative to conventional chemical approaches, electrochemical assembly of metal chalcogenide nanoparticles enables the use of two additional levers for tuning the assembly process: electrode material and potential. In our prior work, oxidative and metal-mediated pathways for electrochemical assembly of metal chalcogenide quantum dots (QDs) into three-dimensional gel architectures were investigated independently by employing a noble-metal (Pt) electrode at relatively high potentials and a non-noble metal electrode at relatively low potentials, respectively. In the present work, we reveal competition between the two electrogelation pathways under the condition of high oxidation potentials and non-noble metal electrodes (including Ni, Co, Zn, and Ag), where both pathways are active. We found that the electrogel structure formed under this condition is electrode material-dependent. For Ni, the major phase is oxidative electrogel, not a potential-dependent mixture of oxidative and metal-mediated electrogel that one would expect. A mechanistic study reveals that the metal-mediated electrogelation is suppressed by dithiolates, a side product from the oxidative electrogelation, which block the Ni electrode surface and terminate metal ion release. In contrast, for Co, Ag, and Zn, the electrode surface blockage by dithiolates is less effective than for Ni, such that metal-mediated electrogelation is the primary gelation pathway.
- This article is part of the themed collections: Celebrating International Women’s Day: Women in Nanoscience and Nanoscale 2022 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...