Photochemical reduction of nanocrystalline maghemite to magnetite†
Abstract
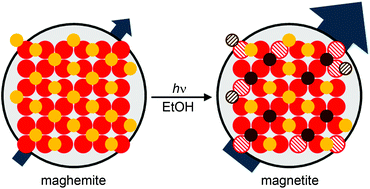
We present a method for thephotochemical conversion of the inverse spinel iron oxides in which the mixed-valent magnetite phase (Fe3O4) is accessed from the maghemite phase (γ-Fe2O3) via a stable, colloidal nanocrystal-to-nanocrystal transformation. Anaerobic UV-irradiation of colloidal γ-Fe2O3 nanocrystals in the presence of ethanol as a sacrificial reductant yields reduction of some Fe3+ to Fe2+, resulting in a topotactic reduction of γ-Fe2O3 to Fe3O4. This reduction is evidenced by the emergence of charge-transfer absorption and increased d-spacing in UV-irradiated nanocrystals. Redox titrations reveal that ∼43% of Fe in <d> = 4.8 nm nanocrystals can be reduced with this method and comparison of optical data indicates similar reduction levels in <d> = 7.3 and 9.0 nm nanocrystals. Addition of excess acetaldehyde during photoreduction shows that the extent of reduction is likely pinned by the hydrogenation of acetaldehyde back to ethanol and can be increased with the use of an alkylborohydride sacrificial reductant. Photochemical reduction is accompanied by increased magnetization and emergence of magnetic features characteristic of Fe3O4. Overall, this work provides a reversible, post-synthetic strategy to obtain Fe3O4 nanocrystals with well-controlled Fe2+ compositions.


 Please wait while we load your content...
Please wait while we load your content...