Multifunctional polyplex micelles for efficient microRNA delivery and accelerated osteogenesis†
Abstract
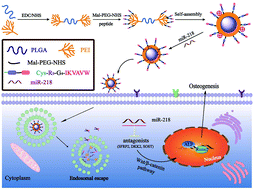
MicroRNAs (miRNAs) are emerging as a novel class of molecular targets and therapeutics to control gene expression for tissue repair and regeneration. However, a safe and effective transfection of miRNAs to cells has been a major barrier to their applications. In this work, a multifunctional polyplex micelle named PPP-RGI was developed as a non-viral gene vector for the efficient transfection of miR-218 (an osteogenic miRNA regulator) to bone marrow-derived mesenchymal stem cells (BMSCs) for accelerated osteogenic differentiation. PPP-RGI was designed and synthesized via conjugation of a multifunctional R9-G4-IKVAVW (RGI) peptide onto an amphiphilic poly(lactide-co-glycolide)-g-polyethylenimine-b-polyethylene glycol (PPP) copolymer. PPP-RGI self-assembled into polyplex micelles and strongly condensed miR-218 to prevent its RNase degradation. When the PPP-RGI/miR-218 complex was brought into contact with BMSCs, it exhibited high internalization efficiency and a fast escape from endo/lysosomes of the BMSCs. Subsequently, miR-218 released from the PPP-RGI/miR-218 complex regulated gene expressions and significantly enhanced the osteogenic differentiation of BMSCs. The multifunctional peptide conjugated nanocarrier serves as an effective miRNA delivery vector to promote osteogenesis.


 Please wait while we load your content...
Please wait while we load your content...