Measuring interactions of DNA with nanoporous protein crystals by atomic force microscopy†
Abstract
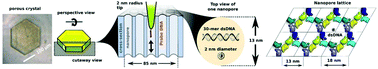
Crosslinked porous protein crystals are a new biomaterial that can be engineered to encapsulate, stabilize, and organize guest molecules, nanoparticles, and biological moieties. In this study, for the first time, the combined interactions of DNA strands with porous protein crystals are quantitatively measured by high-resolution atomic force microscopy (AFM) and chemical force microscopy. The surface structure of protein crystals with unusually large pores was observed in liquid via high-resolution AFM. Force–distance (F–D) curves were also obtained using AFM tips modified to present or capture DNA. The modification of AFM tips allowed the tips to covalently bind DNA that was pre-loaded in the protein crystal nanopores. The modified tips enabled the interactions of DNA molecules with protein crystals to be quantitatively studied while revealing the morphology of the buffer-immersed protein crystal surface in detail, thereby preserving the structure and properties of protein crystals that could be disrupted or destroyed by drying. The hexagonal space group was manifest at the crystal surface, as were the strong interactions between DNA and the porous protein crystals in question. In sum, this study furthered our understanding of how a new protein-based biomaterial can be used to bind guest DNA assemblies.


 Please wait while we load your content...
Please wait while we load your content...