An AlCl3 coordinating interlayer spacing in microcrystalline graphite facilitates ultra-stable and high-performance sodium storage†
Abstract
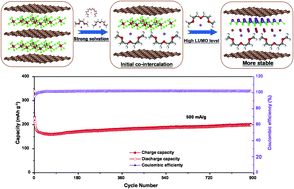
Metal chloride-intercalated graphite intercalation compounds (MC-GICs) show a perfect sandwich structure with high electronic conductivity and chemical stability, but there are few applications for MC-GICs in anode materials of sodium ion batteries (SIBs). Herein, we selected a splendid host microcrystalline graphite (MG) to synthesize an AlCl3 intercalated MG intercalation compound (AlCl3-MGIC) anode material and demonstrated that it is suitable for SIBs via electrolyte optimization. The AlCl3-MGIC electrode is primarily compared in four electrolytes. Sodium storage is proposed for co-intercalation and conversion reactions by simultaneously selecting a compatible NaPF6/diethylene glycol dimethyl ether (DEGDME) electrolyte. As a result, the AlCl3-MGIC anode delivers a specific capacity of 202 mA h g−1 at a current density of 0.2 A g−1 after 100 cycles and still exhibits a satisfactory capacity of 198 mA h g−1 after 900 cycles. Density functional theory calculations further illustrate that DEGDME solvent molecules offer moderate adsorption energy to sodium ions that guarantees structure stabilization of GICs during repeated cycling. This work provides a theoretical basis for designing sodium ion storage with a graphite layered structure and unveiling the prospects of MC-GIC materials as high-performance anodes.


 Please wait while we load your content...
Please wait while we load your content...