Bifunctional single-atomic Mn sites for energy-efficient hydrogen production†
Abstract
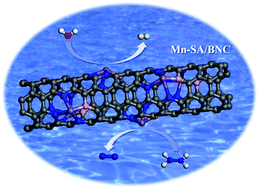
The electrocatalytic hydrogen evolution reaction (HER) for H2 production is essential for future renewable and clean energy technology. Screening energy-saving, low-cost, and highly active catalysts efficiently, however, is still a grand challenge due to the sluggish kinetics of the oxygen evolution reaction (OER) in electrolyzing water. Herein, we present a single atomic Mn site anchored on a boron nitrogen co-doped carbon nanotube array (Mn-SA/BNC), which is perfectly combined with the hydrazine electrooxidation reaction (HzOR) boosted water electrolysis concept. The obtained catalyst achieves 51 mV overpotential at the current density of −10 mA cm−2 for the cathodic HER and 132 mV versus the reversible hydrogen electrode for HzOR, respectively. Besides, in a two-electrode overall hydrazine splitting (OHzS) system, the Mn-SA/BNC catalyst only needs a cell voltage of only 0.41 V to output 10 mA cm−1, with strong durability and nearly 100% faradaic efficiency for H2 production. This work highlights a low-cost and high-efficiency energy-saving H2 production pathway.

 Please wait while we load your content...
Please wait while we load your content...