A combined theoretical/experimental study highlighting the formation of carbides on Ru nanoparticles during CO hydrogenation†
Abstract
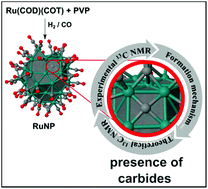
Formation of stable carbides during CO bond dissociation on small ruthenium nanoparticles (RuNPs) is demonstrated, both by means of DFT calculations and by solid state 13C NMR techniques. Theoretical calculations of chemical shifts in several model clusters are employed in order to secure experimental spectroscopic assignations for surface ruthenium carbides. Mechanistic DFT investigations, carried out on a realistic Ru55 nanoparticle model (∼1 nm) in terms of size, structure and surface composition, reveal that ruthenium carbides are obtained during CO hydrogenation. Calculations also indicate that carbide formation via hydrogen-assisted hydroxymethylidyne (COH) pathways is exothermic and occurs at reasonable kinetic cost on standard sites of the RuNPs, such as 4-fold ones on flat terraces, and not only in steps as previously suggested. Another novel outcome of the DFT mechanistic study consists of the possible formation of μ6 ruthenium carbides in the tip-B5 site, similar examples being known only for molecular ruthenium clusters. Moreover, based on DFT energies, the possible rearrangement of the surface metal atoms around the same tip-site results in a μ-Ru atom coordinated to the remaining RuNP moiety, reminiscent of a pseudo-octahedral metal center on the NP surface.


 Please wait while we load your content...
Please wait while we load your content...