The simultaneous adsorption, activation and in situ reduction of carbon dioxide over Au-loading BiOCl with rich oxygen vacancies†
Abstract
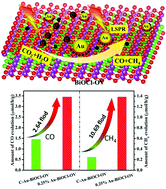
The main process of carbon dioxide (CO2) photoreduction is that excited electrons are transported to surface active sites to reduce adsorbed CO2 molecules. Obviously, electron transfer to the active site is one of the key steps in this process. However, current catalysts for CO2 adsorption, activation, and electron reduction occur in different locations, which greatly reduce the efficiency of photocatalysis. Herein, through a spontaneous chemical redox approach, the plasmonic photocatalysts of Au–BiOCl-OV with enhanced interfacial interaction were fabricated for visible light CO2 reduction through the simultaneous adsorption, activation and in situ reduction of CO2 without a sacrificial agent. By loading gold (Au) on the oxygen vacancy (OV), Au and BiOCl-OV formed a direct and tight interface contact, whose fine structure was confirmed by SEM, TEM, EPR and XPS, which not only effectively boosts the light utilization efficiency and the light carrier separation ability, but also can simultaneously adsorb, activate and in situ reduce carbon dioxide for highly efficient visible light photocatalysis. Thanks to the synergistic influence of Au and OV, Au–BiOCl-OV exhibits excellent photocatalytic performance without sacrificial agent and outstanding stability with a high CO and CH4 production yield, reaching 4.85 μmol g−1 h−1, which were 2.8 times higher than C–Au–BiOCl-OV (obtained by traditional NaBH4 reduction). This study proposes a new strategy for the production of high-performance collaborative catalysis in photocatalytic CO2 reduction.


 Please wait while we load your content...
Please wait while we load your content...