3D porous Ni/NiOx as a bifunctional oxygen electrocatalyst derived from freeze-dried Ni(OH)2†
Abstract
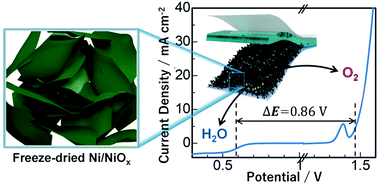
Bifunctional electrocatalytic properties of freeze-dried Ni/NiOx, freeze-dried NiO, and freeze-dried Ni(OH)2 are reported. Freeze-dried Ni(OH)2 was synthesized by the freeze-drying method. Freeze-dried Ni/NiOx and freeze-dried Ni were obtained from the thermal annealing of the material. Both Ni(OH)2 and Ni/NiOx could sustain with freestanding freeze-dried 3D structures without any carbon support. Freeze-dried Ni/NiOx exhibited excellent bifunctional electrocatalytic properties with the ORR performance at 0.62 V (half-wave potential) and OER at 1.47 V (η = 10 mA cm−2). Using freeze-dried metal hydroxides can be considered useful in a wide range of carbon-free applications and can improve the electrocatalytic performance. The bifunctional catalytic activities were calculated to be 0.86, 0.98 and 1.14 V for freeze-dried Ni/NiOx, freeze-dried NiO and freeze-dried Ni(OH)2, respectively. The stacking of 2D sheets into 3D mass seemed to play a vital role behind this excellent bifunctionality of freeze-dried Ni/NiOx. The material reveals possible applications in Zn-air batteries. Besides, the strategy developed herein could be justified to obtain other transition metal-oriented bifunctional electrocatalysts as alternatives to Pt- and Ir/Ru-based expensive benchmark catalysts.
- This article is part of the themed collection: Nanoscale Horizons, Nanoscale, and ChemComm: Nanocatalysis


 Please wait while we load your content...
Please wait while we load your content...