Analysis of dendrimer-protein interactions and their implications on potential applications of dendrimers in nanomedicine
Abstract
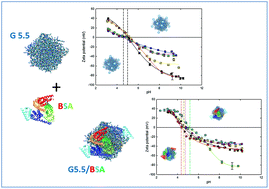
This work addresses how G5.5 PAMAM dendrimers form complexes with bovine serum albumin (BSA). Analytical techniques, such as UV-vis spectrophotometry, dynamic light scattering, electrophoretic mobility, quartz crystal microbalance with dissipation monitoring (QCM-D), circular dichroism (CD), and contact angle were used to analyze the properties of the dendrimers systems. The binding of protein to dendrimers can alter the structure, mobility, conformation and functional activity of the dendrimer. The results show that BSA interactions with G5.5 dendrimer carriers are driven both by electrostatic and hydrophobic forces. Dendrimer surface charge is reduced upon contact with the protein. The protein shell formed on the surface of the carrier is very stable as evidenced by the QCM-D measurements. On the other hand, the CD spectra indicates a change in the secondary structure of the protein. The size of the changes is significantly dependent on the ratio of protein to dendrimer. Understanding the mechanism of interaction of potential carriers with proteins is important for their internalization into the cell.


 Please wait while we load your content...
Please wait while we load your content...