Abstract
Covering: up to 2021
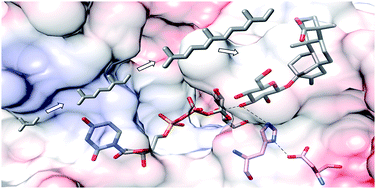
Terpenoids are physiologically active substances that are of great importance to humans. Their physicochemical properties are modified by glycosylation, in terms of polarity, volatility, solubility and reactivity, and their bioactivities are altered accordingly. Significant scientific progress has been made in the functional study of glycosylated terpenes and numerous plant enzymes involved in regio- and enantioselective glycosylation have been characterized, a reaction that remains chemically challenging. Crucial clues to the mechanism of terpenoid glycosylation were recently provided by the first crystal structures of a diterpene glycosyltransferase UGT76G1. Here, we review biochemically characterized terpenoid glycosyltransferases, compare their functions and primary structures, discuss their acceptor and donor substrate tolerance and product specificity, and elaborate features of the 3D structures of the first terpenoid glycosyltransferases from plants.


 Please wait while we load your content...
Please wait while we load your content...