C2 weakens the turnover frequency during the melting of FexCy: insights from reactive MD simulations†
Abstract
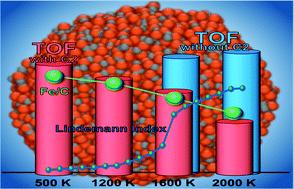
The first-order phase transition plays a pivotal role in material behaviors, yet that of carbides, a type of important material, has not been systematically studied. Herein, the melting process and structural properties of binary iron carbide (FexCy) nanoparticles are characterized by reactive molecular dynamics simulation. It was found that the melting point of FexCy nanoparticles decreased with the decreasing size and increased with the increasing Fe/C ratio, which were consistent with the experimental results. The melting process starts at the surface and proceeds inwards. The carbon atoms are fully activated before reaching the melting point and the iron core melts last. At high temperatures, carbon atoms exhibit significant outward diffusion behavior and form carbon deposition on the surface. When the temperature exceeds the pre-melting point, although the high temperature gives the nanoparticles more atomic active sites with low coordination, the surface carbon accumulation, such as C2, blocks the active sites leading to a lower turnover frequency of FexCy for CO dissociation. These findings provide an atomistic comprehension of the melting mechanisms and behaviors of binary FexCy nanoparticles, as well as a theoretical foundation for understanding their structural transformation as a catalyst, which is caused by the heat released from catalytic exothermic reactions.


 Please wait while we load your content...
Please wait while we load your content...
