Minisci aroylation of N-heterocycles using choline persulfate in water under mild conditions†
Abstract
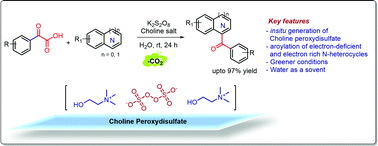
Metal persulfate mediated thermal oxidative organic transformations invariably require a higher temperature and frequently use an organic solvent. The objective of this work was to develop persulfate mediated oxidative transformations that can be performed nearly at room temperature using water as a solvent. This report describes modified Minisci aroylation of isoquinolines with arylglyoxylic acids using choline persulfate and its pre-composition (choline acetate and K2S2O8) in water at 40 °C. A few other nitrogen heterocycles were also utilized affording various aroylated products in good to excellent yields. Unlike metal persulfate that could produce metal salt byproducts, a key feature of the chemistry reported herein includes the use of environmentally benign choline persulfate containing biodegradable choline as a counter-cation, the Minisci reaction demonstrated at 40 °C in water as the only solvent, and unconventional activation of persulfate.


 Please wait while we load your content...
Please wait while we load your content...