Visible-light-promoted/PIFA-mediated direct C–H acylation of quinoxalin-2(1H)-ones with aldehydes†
Abstract
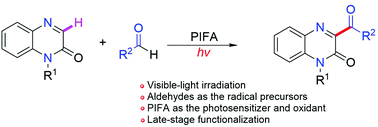
With aldehydes as the radical precursors under visible-light irradiation, a simple and mild C–H acylation reaction of quinoxalin-2(1H)-ones has been achieved. A wide variety of functional groups could be incorporated into the products by employing diverse aldehydes and quinoxalin-2(1H)-one derivatives. Also, this method was applied to the modification of natural molecules and pharmaceutically relevant compounds. A mechanistic study revealed that an HAT process occurred in the acylation reaction for the generation of acyl radicals, and that PIFA ([bis(trifluoroacetoxy)iodo]benzene) acted both as the photosensitizer and oxidant in this reaction.


 Please wait while we load your content...
Please wait while we load your content...