The reactivity of Nbn+ clusters with acetylene and ethylene to produce a cubic aromatic metal carbide Nb4C4+†
Abstract
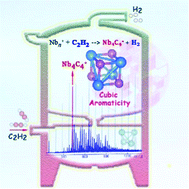
The study of clusters with strongly size-dependent properties has attracted extensive attention due to their potential applications in building new materials. Utilizing a home-made multiple-ion laminar flow tube reactor in tandem with a customized triple quadrupole mass spectrometer, we have studied the reactions of niobium cationic clusters with acetylene and ethylene. It is observed that dehydrogenation dominates the reaction process, and Nb4C4+ shows up prominently in the mass spectra. Quantum chemistry calculations reveal that the most stable structure of Nb4C4+ corresponds to a slightly distorted cubic structure with D2d symmetry, with reasonable stability pertaining to its cubic aromaticity. We illustrate the dehydrogenation reaction paths of Nb4+ + 2C2H2 to form the prominent Nb4C4+ product, along with the highly exothermic reactions to produce H2.


 Please wait while we load your content...
Please wait while we load your content...