Crystal structure of 1-(2,4,6-trichlorobenzoyloxy) benzotriazole (TCB-OBt): observation of uncommon intermolecular oxygen–oxygen interaction and synthetic application in amidation†
Abstract
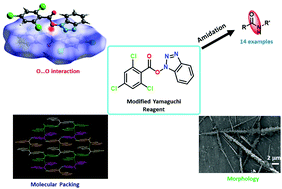
Herein, we investigated the supramolecular assembly of a modified Yamaguchi reagent TCB-OBt. Interestingly, each molecule is interconnected through novel chalcogen–chalcogen (O⋯O) interaction, π–π stacking, and aromatic C–H⋯O interaction. Hirshfeld surface analysis confirmed the existence of uncommon O⋯O interactions. A well-organized supramolecular layer structure and helical arrangement were observed in the crystal structure. TCB-OBt crystallized in the O-substituted desmotropic form. DFT calculations suggest that the O-substituted form is more stable than the N-substituted form (TCB-(N)-OBt). Morphology analysis indicates the formation of a fantastically well organized, continuous block-shaped system. Furthermore, the designed reagent works as an efficient activating reagent for amide bond formation with good yields under mild reaction conditions. Use of this reagent avoided intractable acid chlorides, and this new mixed-anhydride-based reagent may further be applicable for many other organic transformations.


 Please wait while we load your content...
Please wait while we load your content...