A dendrimer-functionalized turn-on fluorescence probe based on enzyme-activated debonding feature of azobenzene linkage
Abstract
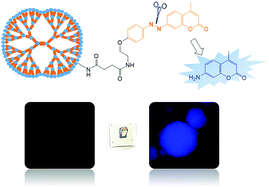
The hypoxic feature of tumors has led to researchers developing hypoxia-activated prodrugs and probes that leverage oxidoreductases overexpressed in tumor tissues. For example, hypoxia causes overexpression of NADPH-quinone oxidoreductase 1 (NQO1), a two-electron reductase. Tumorspheres are also hypoxic, meaning that they are a useful in vitro platform for developing NQO1-directed chemotherapeutic drugs. This study proposed a method of synthesizing a NQO1-expression-detecting functional poly(amido amine) (PAMAM) dendrimer peripherally grafted with nonfluorescent coumarin probes through the azo linkage. Blue-fluorescent 7-amino-4-methylcoumarin (AMC) was used as the model probe molecule. The fluorescence of the macromolecular probe was turned on through reductive activation. The aromatic azo linkers were engineered to be recognized and cleaved by NQO1, resulting in the release of probe molecules and turning on coumarin fluorescence. Fluorogenic and colorimetric analysis indicated that the dendrimers modified with AMC could trigger NQO1 reduction. A549 tumorspheres were found to exhibit intense blue fluorescence when treated with the dendrimers, indicating that AMC probes had escaped from the dendrimers. The results implied that the high NQO1 expression in A549 tumorspheres led to bright and high-contrast fluorescence images. The proposed method for synthesizing probe-containing dendrimers is facile, and the compound can be employed in tumor diagnosis.


 Please wait while we load your content...
Please wait while we load your content...