Fluorescent chemosensors for Hg2+ ions based on a pyridine-attached phenanthridine probe†
Abstract
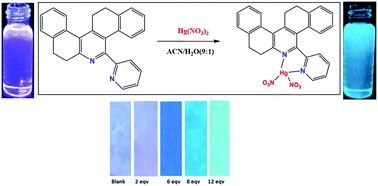
A ratiometric fluorescence phenanthridine sensor (4), functionalised with a pyridine moiety, has been prepared. 4 displayed both fluorescence and absorbance changes for the selective recognition of Hg2+ ions in acetonitrile/water as a solvent in the presence of other relevant interfering metal ions. 4 displayed a noticeable color change of blue to green under exposure to UV light at 365 nm. The binding constant value (Ka) was found to be 2.6198 × 108 M−1 for Hg2+ ions. The probe 4 showed a high selectivity and sensitivity for Hg2+ ions over other interfering competing cations. The ligand 4 interacted with Hg2+ ions to create a 1 : 1 complex, and 1H NMR titration and Job's plot studies further confirmed this mechanism. At the same time, 4 exhibits a high sensitivity to Hg2+ with a detection limit of 49.87 nM. The sensing can be carried out in a wide pH range of 3 to 11. In addition, the sensor 4 can be applied to living bacterial cells for ratiometric visual monitoring of Hg2+ ions by bioimaging. The real time analysis of 4 was also effectively verified by the determination of Hg2+ ions using a paper strip test kit and in different environmental water samples.


 Please wait while we load your content...
Please wait while we load your content...