Direct synthesis of 4-hydroxycoumarins and 4-hydroxy-6-methyl-2-pyrone containing chroman-4-ones via a silver catalyzed radical cascade cyclization reaction†
Abstract
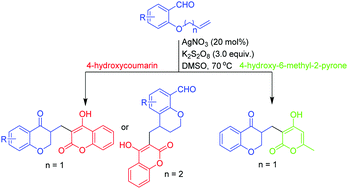
For the first time, we report a novel silver catalyzed radical cascade cyclization reaction of 2-(allyloxy)arylaldehydes to synthesize two new libraries of chroman-4-ones by using 4-hydroxycoumarins and 4-hydroxy-6-methyl-2-pyrone as radical precursors. AgNO3/K2S2O8 acts as an efficient oxidizing system and smoothly drives the reaction producing the 4-hydroxycoumarins and 4-hydroxy-6-methyl-2-pyrone containing chroman-4-ones in moderate to good yields. The protocol also makes different chemoselective products while using 2-(but-3-en-1-yloxy)benzaldehydes as radical acceptors. We have confirmed the participation of radicals by performing various radical trapping experiments with 2,2,6,6-tetramethylpiperidine-1-oxyl, butylated hydroxytoluene, and diphenyl ethylene.


 Please wait while we load your content...
Please wait while we load your content...