Recent developments in synthesis of catechols by Dakin oxidation
Abstract
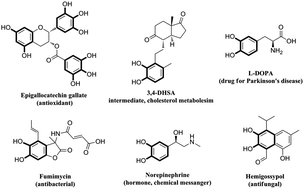
In recent years, Dakin oxidation has evolved primarily around the conversion of ortho- and para-hydroxy benzaldehydes and acetophenones to dihydric phenols, which are found in nature and complex organic materials. In the traditional Dakin reaction, an excess amount of NaOH was used along with hydrogen peroxide. The synthetic community has devoted much time and effort to developing efficient basic, acidic and neutral reaction media for Dakin oxidation. The use of in situ generated H2O2 and molecular oxygen was also tested as an alternative oxidant for this reaction. In this review, we will discuss recent developments in Dakin oxidation with representative examples as well as future challenges and potential applications for this exciting field.
- This article is part of the themed collection: 2021 Focus and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...