Novel coumarin–pyrazoline hybrids: synthesis, cytotoxicity evaluation and molecular dynamics study
Abstract
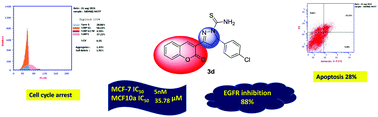
A novel series of coumarin–pyrazoline hybrids 3a–f, 4a–c and 5a–c have been synthesized and tested for their antiproliferative activity against the breast cancer cell line MCF-7. The most active compounds 3d, 3e, 3f, 5a and 5c were also evaluated for their ability to inhibit EGFR expression with reference to erlotinib. In silico studies using rigid docking, flexible docking and molecular dynamics were performed to explore the possibility of direct interactions between the active molecules and the ATP-binding site of EGFR. Most compounds demonstrated a potent cytotoxic activity against the MCF-7 cell line. The most active compounds 3d, 3e, 3f, 5a and 5c with IC50 values of 5, 26, 44, 20, and 50 nM, respectively, were further tested against HCT-116, HepG-2, A549 and SGC-7901 cell lines. All the tested compounds showed better activity than the reference standard drugs (doxorubicin and erlotinib) in all the tested cell lines. Compound 5a was the most potent one against HCT-116 with an IC50 value of 5 nM, while compound 3d was the most potent one against the breast cancer cell line MCF-7, liver HepG-2, lung A549 and the gastric cancer cell line SGC-7901 with IC50 values of 5, 77, 27 and 60 nM, respectively. Compounds 3d and 5a were tested for their cytotoxic effects on the normal breast cancer cell line MCF10a and their IC50 values were 35.78 and 22.77 μM, respectively, indicating good selectivity. The most active compounds 3d, 3e, 3f, 5a and 5c exhibited percent reduction in EGFR level ranging from 80.9 to 88.0%. The apoptotic effect of compounds 3d and 5a on MCF-7 cells was investigated through cell cycle analysis. Both compounds showed increases in the number of cells in the pre-G1 phase of 14 and 22 folds, respectively, compared to the control. Both compounds exhibited total apoptosis of 28.06 and 43.88%, respectively. Docking of the new ligands revealed high scores compared to that of erlotinib. In the case of thiourea derivatives 3d and 3e, more stable hydrogen bonds via the thiourea group were demonstrated through molecular dynamics.


 Please wait while we load your content...
Please wait while we load your content...