Synthesis and properties of 1,2,3-diazapnictol-5-yl substituted ferrocenes†
Abstract
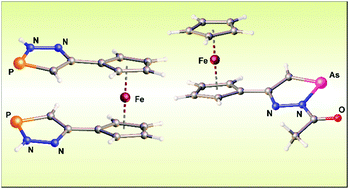
The reaction of acetylferrocene hydrazone or 1,1′-diacetylferrocene dihydrazone with PCl3 gives the corresponding azines as the only organometallic product. The separation of the hydrazone function with a 1,4-phenylene or propane-2,2-diyl spacer enables the preparation of new heterocyclic compounds [(2H-1,2,3-diazaphosphol-5-yl)-4-phenyl]ferrocene (2b) and [1-(2H-1,2,3-diazaphosphol-5-yl)-1-methyl-ethyl]ferrocene (2c) but in low yields. Using acetylated hydrazones, that do not undergo transformation into stable azine molecules, led to the synthesis of (2-acetyl-1,2,3-diazaphosphol-5-yl)ferrocene (5a) and 1,1′-bis(2-acetyl-1,2,3-diazaphosphol-5-yl)ferrocene (8) in up to 70% yield. N-Acylated compounds 5a and 8 are readily converted to (2H-1,2,3-diazaphosphol-5-yl)ferrocene (2a) and 1,1′-bis(2H-1,2,3-diazaphosphol-5-yl)ferrocene (9). Analogous reactions were utilized for the preparation of (2-acetyl-1,2,3-diazarsol-5-yl)ferrocene (10), (2H-1,2,3-diazarsol-5-yl)ferrocene (11), 1,1′-bis(2-acetyl-1,2,3-diazarsol-5-yl)ferrocene (12) and 1,1′-bis(2H-1,2,3-diazarsol-5-yl)ferrocene (13). Electrochemical studies on a series of new ferrocenes showed that the 2H-1,2,3-diazapnictol-5-yl substituent possesses weak electron-withdrawing ability which is enhanced by acetylation in the 2-position of such a heterocycle. We also observed the electroactivity of the 1,2,3-diazapnictolyl substituent as it could be irreversibly reduced at potentials from −1.97 to −2.93 V vs. the Fc0/+ couple. All prepared compounds have been fully characterized using spectroscopic methods and molecular structures of 2a, 2b, 2c, 5a, 8, 9 and 10 are reported.


 Please wait while we load your content...
Please wait while we load your content...